Pictures of the Day CH320M/CH328M
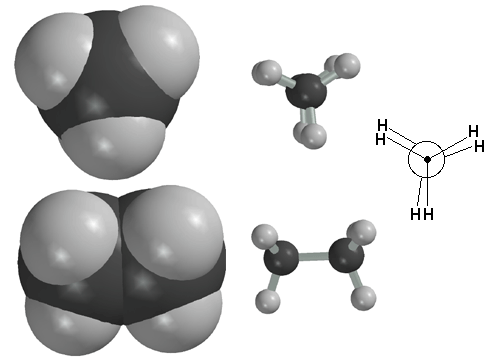
Here is ethane in the staggered conformation. Shown also is the Newman projection of the same ethane molecule. Remember, the circle represents the front carbon atom that is seen when the molecule is viewed down the carbon-carbon sigma bond. Ethane spends most of its time in a staggered conformation, because this is the most stable conformation.
You can see some movies we have made that show how bond rotation in alkanes is really the result of bond vibrations. At any given instant, the molecule is vibrating, and since there is very little rotation barrier around the C-C sigma bond, random rotation occurs largely as a consequence of the vibrations. Note that although very dynamic, the molecule spends the majority of its time in the staggered conformation, because that is lower in energy.
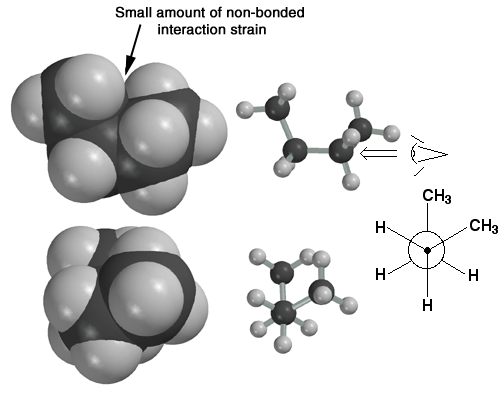
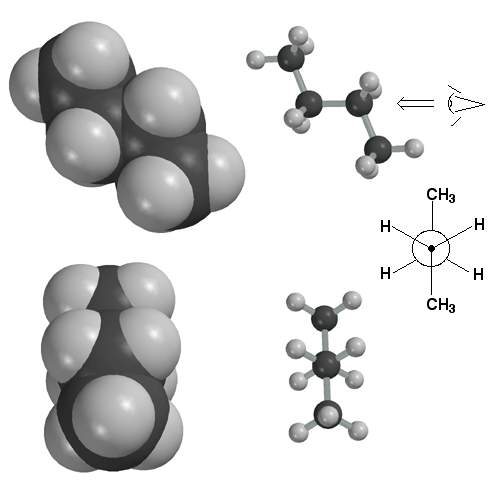
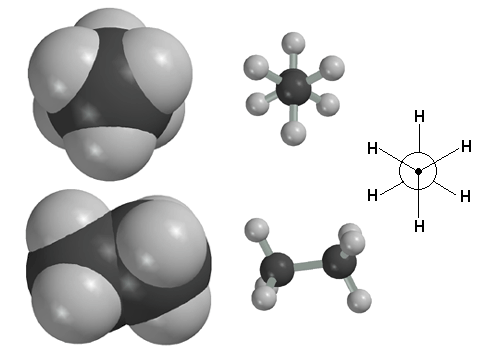
Here the butane molecule is in the anti conformation. The Newman Projection at the bottom clearly shows that this conformation maximizes the distance between the methyl groups. This, in turn, minimizes steric strain and makes the butane molecule most stable in the anti-conformation. The Newman projection is drawn from the perspective indicated by the "eye".
You can see some movies we have made that show how bond rotation in alkanes is really the result of bond vibrations. At any given instant, the molecule is vibrating, and since there is very little rotation barrier around the C-C sigma bond, random rotation occurs largely as a consequence of the vibrations. Note that although very dynamic, the molecule spends the majority of its time in the staggered-anti conformation, because that is lowest in energy.
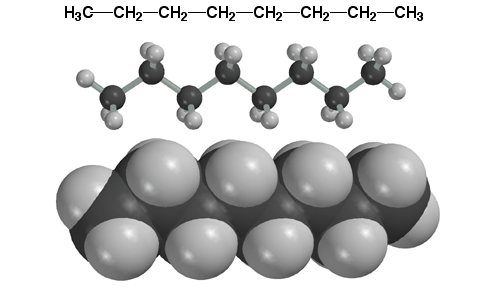
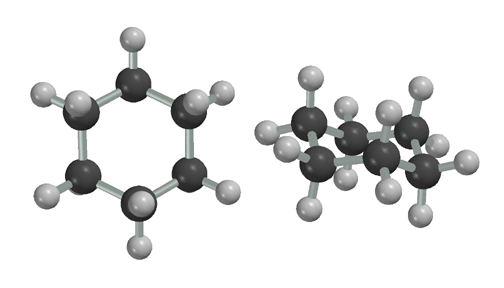
Here is a picture of octane in the staggered, anti conformation. Staggered, anti is the preferred conformation of alkanes because both torsional and steric strain are minimized. It is important to remember that at any given instant at room temperature, the molecule is likely to be bent while vibrating and rotating. It is only the TIME-AVERAGED view that is being represented by the entirely staggered, anti conformation. Also, the molecule will be in the entirely staggered, anti conformation near absolute zero temperature, where there is not enough energy to vibrate and thus rotate bonds. The preference for the staggered, anti conformation is why we use the zig-zag represention for alkanes.
You can see some movies we have made that show how bond rotation in alkanes is really the result of bond vibrations. At any given instant, the molecule is vibrating, and since there is very little rotation barrier around the C-C sigma bond, random rotation occurs largely as a consequence of the vibrations. Note that although very dynamic, the molecule spends the majority of its time in the staggered-anti conformation, because that is lowest in energy.
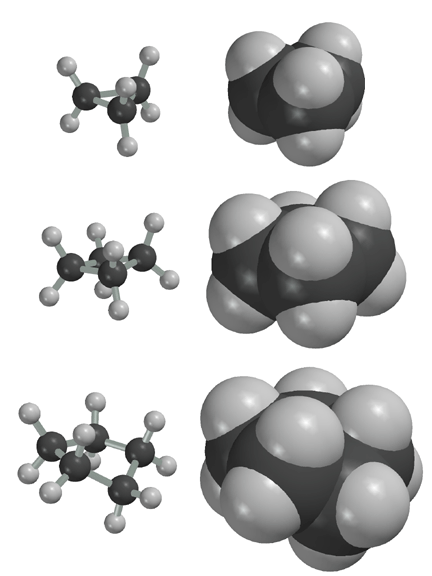
Cyclopropane is a 3-carbon ring structure. The bond angles form an equilateral triangle with bond angles of 60 degrees. The bond angles between the carbon atoms would prefer to be 109.5 degrees but because of geometrical constraints of the 3 carbon ring, this is not possible. Thus, there is considerable angle strain in cyclopropane. There is no non-bonded interaction strain in cyclopropane because no atoms crash into one another. but there is significant torsional strain in cyclopropane because the bonds are eclipsed, causing electron repulsion. You can see the torsional strain if you draw Newman projections for any of the C-C bond in cyclopropane.
Cyclobutane is more stable than cyclopropane. Cyclobutane has considerable angle strain, but not as much as in cyclopropane. Unlike cyclopropane, which is flat, cyclobutane puckers to lessen somewhat (not eliminate, however) torsional strain. Puckering allows the bonds to remain only partially eclipsed. There is no evidence of non-bonded interaction strain for cyclobutane, as can be seen by inspecting the space-filling model.
Cyclopentane has little angle strain because the interior angles of a pentagon are 108 °. Like cyclobutane, cyclopentane is able to pucker, making the bonds only partially eclipsed, and thereby relieving some, but not all, of its torsional strain. There is no evidence of non-bonded interaction strain for cyclopentane, as can be seen by inspecting the space-filling model.