Pictures of the Day CH320M/CH328M
9-17-25
This
is cyclohexane in the chair conformation. This is the most
stable conformation for cyclohexane. Chair cyclohexane has no angle strain,
as the bond angles around carbon are very close to the ideal 109.5°. Notice also how
all the bonds are perfectly staggered, thereby eliminating torsional strain.
As can be seen in the space filling model, there is no appreciable non-bonded interaction
strain in unsubstituted chair cyclohexane. Non-bonded interactions strain
is only introduced when an axial hydrogen atom is replaced by a larger atom
or group.
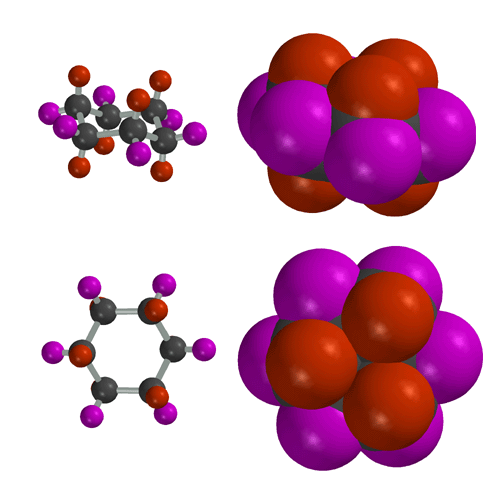
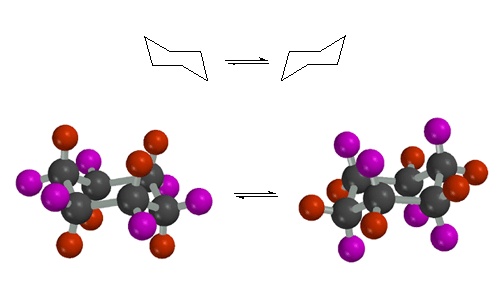
Side
(Above) and Top (Below) views of chair cyclohexane. Chair cyclohexane has
two distinct types of hydrogen atoms, axial (red) and equitorial (purple).
The axial positions point in a direction that is normal to the mean plane
of the cyclohexane ring, while the equatorial positions are splayed out around
the periphery of the ring. You need to be able to recognize the difference
between axial and equatorial positions, and you need to be able to draw chair
cyclohexane on a piece of paper.
Here
are the two chair conformations of cyclohexane, with the hydrogen atoms colored
to indicate the axial and equatorial positions. For the chair on the left,
the axial hydrogens are drawn red, while the equatorial hydrogens are drawn
purple. After the ring flips to the other chair, shown on the right, notice
how the axial and equatorial hydrogens have exchanged.
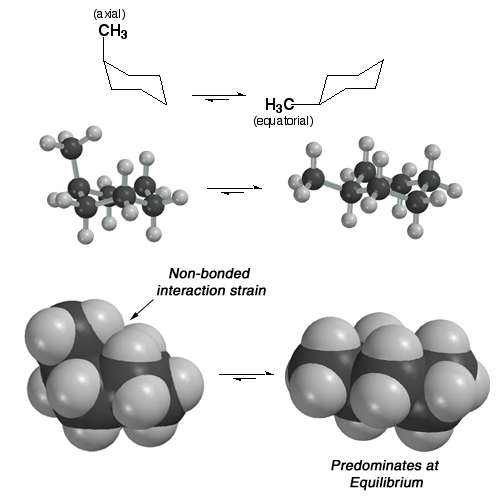
Steric
Strain (Also Called Non-bonded Interaction Strain) in Methyl
Cyclohexane: 1,3 Diaxial Strain
Groups
larger than hydrogen such as the methyl group shown here experience more non-bonded
interaction strain (also callled steric strain, i.e. atom "crunching") in the axial position (1,3-diaxial
interactions) than in the equatorial position. As a result, the chair on
the right with the methyl group equatorial predominates at equilibrium. The
larger the group, the greater its preference for being equatorial.