Pictures of the Day
3-26-2024
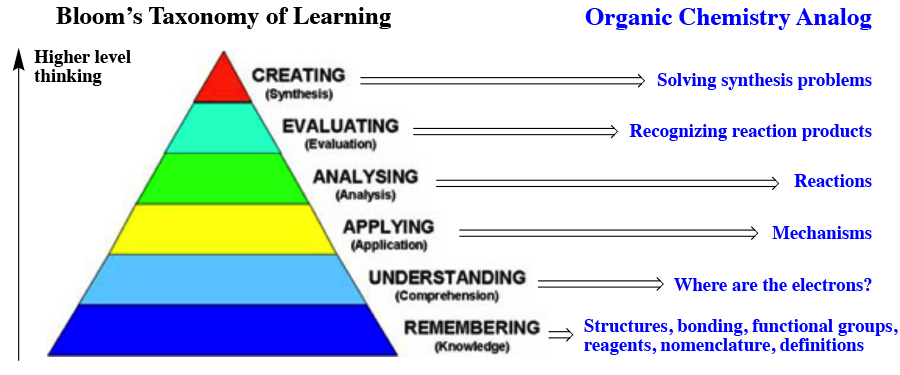
Organic chemistry is difficult because it requires higher order thinking. According to Bloom's taxonomy of learning, the lowest level of learning involves pure memorization ("Remembering") As one moves up the pyramid to higher learning, understanding, applying, analysing, evaluating and creating are reached. I believe there are Organic chemistry analogs of all of these, culminating in synthesis which inolves creativity along with all of the other levels of thinking. It is likely that many of you have never been challenged all the way to the top of the Bloom's taxonomy of learning pyramid before, explaining why this feels different and disorienting. DO NOT GIVE UP. As shown on the right, we have created tools to help you master each step up the ladder. On the above diagram you can cllick on the tools listed to go directly to them. Also, if you have any questions about how to study, click here to read about the way I learned to study. I never earned a grade lower than an A after I started using this method during my own college career.
I understand that most of you are headed to the health professions, so you may be wondering if mastering synthesis problems will be important for you. I assert that it is. Solving a synthesis problem involves the detailed evaluation of a complex molecule while looking for KREs, then working backwards to the starting materials by analyzying possible reactions involved by thinking through your roadmaps, possibly applying your understanding of mechanism to make sure you predict the correct product for each reaction. This is the exact type of thinking you will need to diagnose a patient. A patient will present various complex combinations of symptoms, then you must evaluate which of these are important, then analyze, apply and understand how the patient got that way and how to get them back to their starting state (healthy) again. In other words, you will learn the "KREs of diagnosis" then work backwards to understand what happened to the originally healthy patient! Therefore, learning how to solve synthesis problems will teach you how to use higher level thinking skills, exactly the kind you will need to develop as a health care professional!
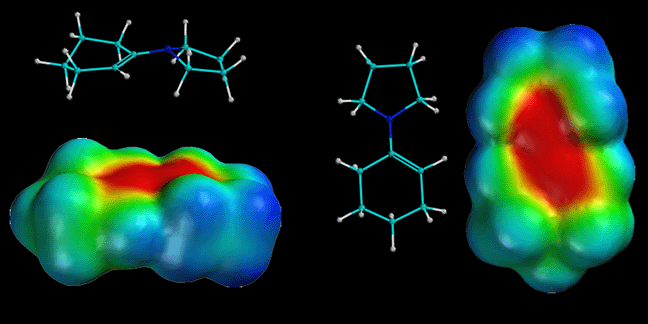
Once Again, A Movie Ripping Off Chemistry
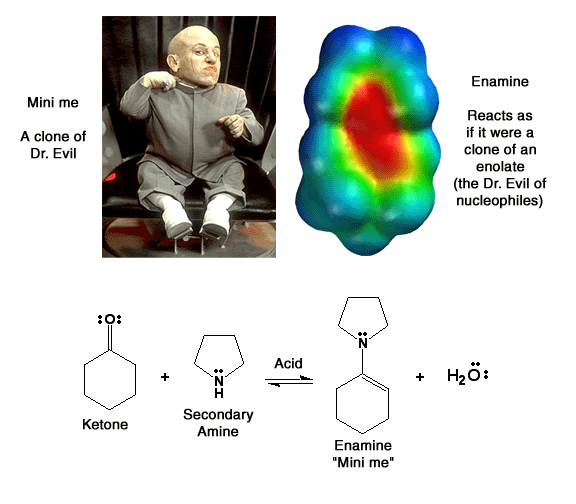
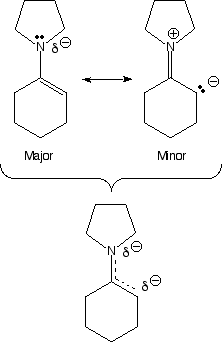
Enamines ("Mini me") Do you believe me now?