Click here for a printer friendly pdf version of all of the Golden Rules pages
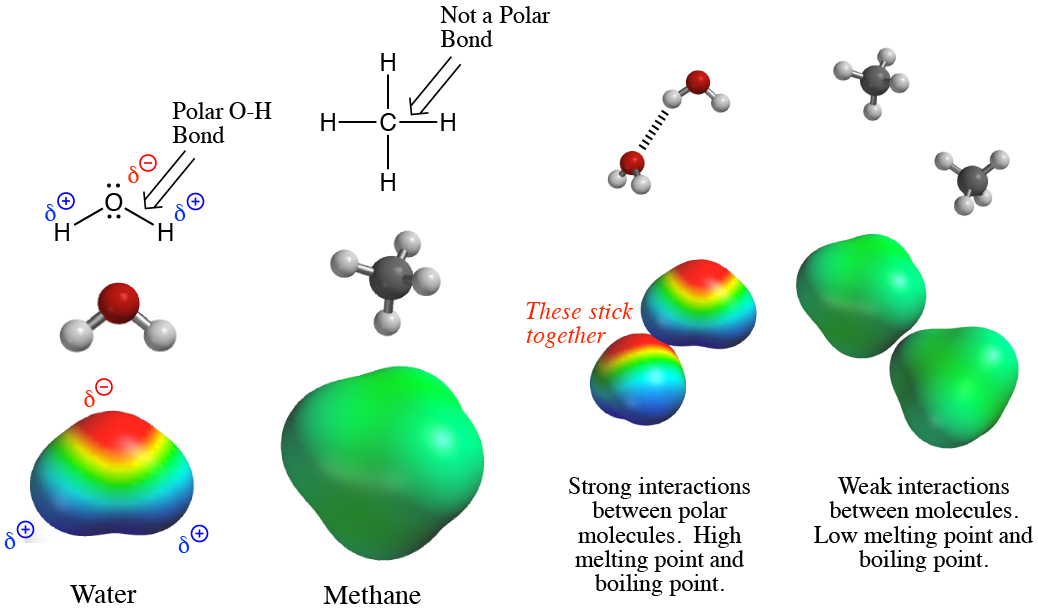
3. The most important question in organic chemistry is "Where are the electrons?" The answer is that electrons are generally in higher amounts around the more electronegative atoms (e.g. F, Cl, O, N). The electronegative atoms pull electron density away from the less electronegative atoms (e.g. C, H) to which they are bonded. Thus, understanding electronegativity provides a simple method of deciding which portions of a molecule have relatively high electron density, and which portions have relatively low electron density. Understanding electron density distributions in molecules, in other words where the electrons are, allows the prediction of most molecular properties and reactions because one of the key principles of organic chemistry is that molecules with relatively electron-rich and/or electron-poor areas have important and characteristic properties and patterns of reactivity.