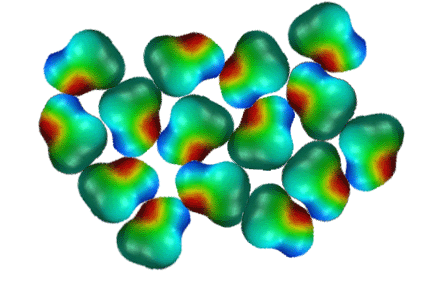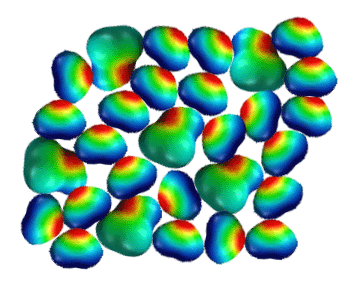Polar
Protic Solvents
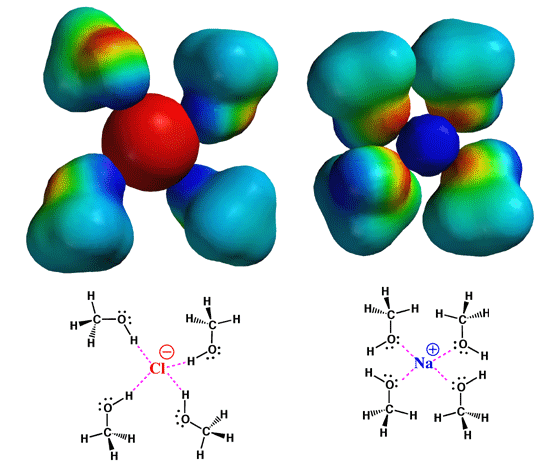
Polar
protic solvents have polar bonds (usually C=O, OH, or NH polar bonds) and
a proton that can take part in hydrogen bonding. A good example is methanol.
As the electrostatic potential surface indicates, there is a center of high
electron density (indicated by the red color) associated with the lone pairs
on oxygen, and a center of low electron density (indicated by the blue color)
associated with the hydroxyl group proton. As a result, methanol and other
polar protic solvents are good a solvating, and thus dissolving ionic compounds.
Here is shown in schematic form how BOTH an anion (red sphere) and cation
(blue sphere) are well solvated by methanol. The anion is surrounded by the
protons of several methanol molecules, while the cation is surrounded by
the lone pairs. If the anion were going to take part as a nucleophile in
an SN2 process, then this strong solvation would decrease
its nucleophilic character, since all of these methanol molecules would have
to move out of the way prior to reaction.
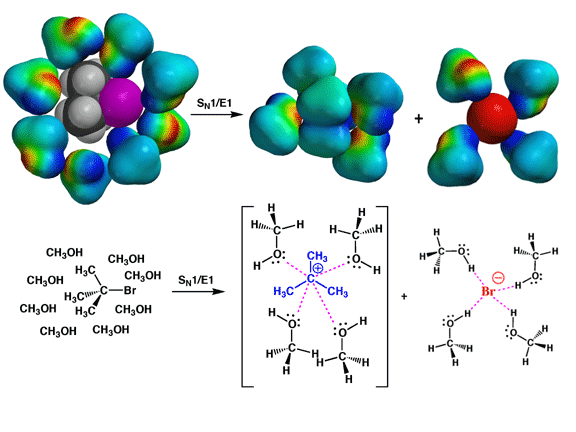




Pictures of the Day CH320M/CH328M
11-10-25
Shown
above are shown solvation in schematic form of how methanol would solvate the tert-butyl
bromide starting material (the tert-butyl bromide is shown colored
by atom type, not electrostatic potential), then on the right how the tert-butyl
cation intermediate and bromide anion leaving group would both be well solvated
by methanol (here the carbocation intermediate and bromide anion are shown
colored by electrostatic potential surface to emphasize how charges dictate
solvation). In SN1 and E1 reactions, the slow step is
formation of a carbocation and leaving group anion. Conditions that favor formation
of these ions will also help facilitate the SN1 and E1
processes. Polar protic solvents like carboxylic acids, alcohols, and water
accelerate SN1/E1 reactions because they are so good
at solvating both the carbocations and leaving group anions that they actually
assist in the process. Because these three solvents are also very weak bases,
they do not promote E2 processes for tertiary alkyl halides. Thus the combined
effects of acceleration of the slow bond cleavage step and the lack of basic
character mean that SN1/E1 processes predominate for
tertiary alkyl halides in carboxylic acids, alcohols, and water.
Hydrogen
Bonding of Alcohols
Alcohols
have relatively high boiling points because the molecules stick together
due to hydrogen bonding. Hydrogen bonding
occurs when the hydrogen atoms attached to N, O, or S atoms interact with
the lone pairs of electrons on N, O, or S atoms. This is really an electrostatic
interaction in that the hydrogen atoms possess a partial positive charge
are thus attracted to the partial negative charge of the electronegative
N, O, or S atoms. For calibration, hydrogen bonds are worth about 1-4 kcals/mol.
Hydrogen bonds are extremely important in almost all aspects of biochemistry
and biology. In the above 2-dimensional illustration, notice how the alcohol
molecules are arranged to produce the maximum amount of hydrogen bonding.
Hydrogen bonds are indicated as red dashed lines in the schematic on the
right.
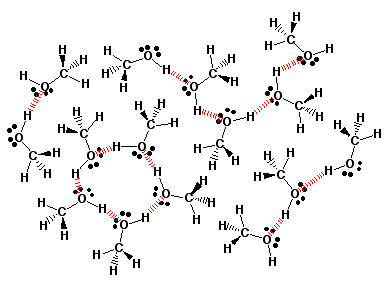
Methanol
Dissolved in Water
Pentane
and Water Don't Mix
Hydrocarbons,
like pentane are not polar and cannot hydrogen bond.
They therefore do not have any way to disrupt the strong hydrogen bonding
between water molecules. Since they cannot disrupt the strong attractions
between water molecules, the non-polar pentane molecules are kept out
of the water and are left to interact with themselves through much weaker
dispersion forces. As a result, “oil and water” do not mix,
and being less dense (carbon has a lower atomic weight than oxygen),
hydrocarbons such as crude oil float on top of water. Again notice the
extensive hydrogen bonding taking place between water molecules in this
2-dimensional illustration.
Solvents
dissolve molecules like themselves (“like
dissolves like”. Thus, hydrogen bonding solvents like water will
dissolve hydrogen bonding substances like alcohols with small carbon
chains because the hydrogen bonds between water molecules is replaced
with hydrogen bonds between the alcohol and water molecules. Notice how
each OH group of the “dissolved” alcohol molecules in this
2-dimensional illustration is taking part in hydrogen bonding with water
molecules.