Pictures of the Day CH320M/CH328M
9-22-25
A
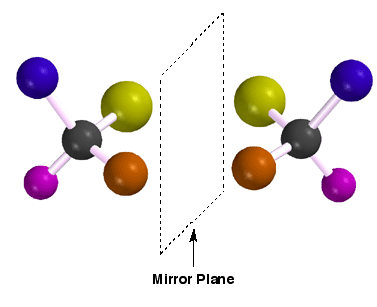
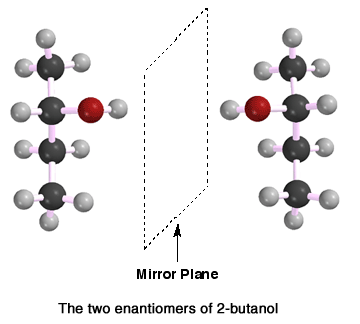
pair of enantiomers is a pair of molecules that are non-superimposable mirror
images of each other. Shown here are a pair of imaginary enantiomers, with
different colors representing different atoms or groups attached to the carbon
chiral center. The point being illustrated is that a tetrahedral carbon atom
with four different atoms or groups attached will almost always be chiral
and thus, will have one enantiomer (non-superimposable mirror image). This
is a consequence of geometry. Two enantiomers will have identical properties
except those properties distinguished by chirality (i.e. rotation of plane
polarized light, taste, smell, etc.). Recall that each enantiomer will rotate
plane polarized light an equal magnitude, but opposite direction.
Chirality
and Tetrahedral Atoms - Enantiomers
Chirality
and Molecules
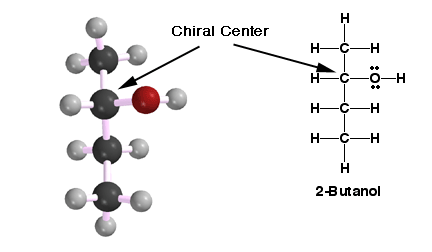
Shown
here is a chiral molecule. It is chiral because it does not have a plane
of symmetry. In other words, there is no plane that can be strategically
placed through the molecule that will show equal reflections on either side
of the plane . This IS different from a mirror plane. A plane of symmetry
can exist inside the molecule whereas a mirror plane is placed outside the
molecule in question. Every molecule can have a mirror image of itself but
the real question remains... is an object superimposable on its mirror image?
If an object is superimposable on its mirror image and it has a plane of
symmetry than it is not chiral. This molecule is chiral because it contradicts
both statements. It is important to notice also that the molecule does have
a carbon atom stereocenter. This is not an absolute determination of chirality
but it is an indication that chirality may be present. Remember this definition
in your stereoisomer arsenal, this molecule and its mirror image are enantiomers.
Achiral
Molecules-They Have Planes of Symmetry
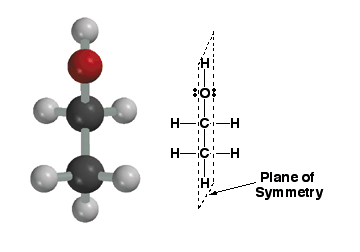
This
molecule, ethanol, is achiral (not chiral) because it posesses a plane of
symmetry. A plane of symmetry is a plane that cuts through a molecule such
that one half of the molecule is a perfect reflection of the other half of
the molecule. The plane of symmetry in this molecule is represented by the
dashed line. Note that this molecule also does not have a chiral center.
Almost (but NOT always), a molecule needs a chiral center to be chiral. Remember,
when looking for planes of symmetry, you are allowed to choose the most symmetric
conformation and temporarily ignore the concepts you have learned about conformational
preference. Don't worry about relative conformational energy when looking
for symmetery. Because enthaonl is not chiral, its mirror image is not a
different stereoisomer, rather it is the same molecule (therefore superimposable
on its own mirror image).
Enantiomers
and Diasteromers - Molecules With Two Chiral Centers
These
structures match the structures in the back of the web
handout on stereochemistry, so you should refer to that.
This molecule, 2,3-dihydroxybutanoic acid, has two chiral centers (one at
carbon 2, and one at carbon 3, so there are four possible stereoisomers.
The 2R,3R and 2S,3S stereoisomers are enantiomers of each other, and the
2R,3S and 2S,3R are enantiomers of each other. These are enantiomer pairs
becuase they are nonsuperimposable mirror images of each other. Any other
pair, such as 2R,3R and 2S,3R, are diastereomers of each other, since they
are stereoisomers that are not enantiomers (not mirror images of each other).
Thus, you use the terms "enantiomers" or "diastereomers" to
describe the stereochemical relationship when comparing different stereoisomers
Meso
Compounds -Molecules with Chiral Centers AND Planes of Symmetry
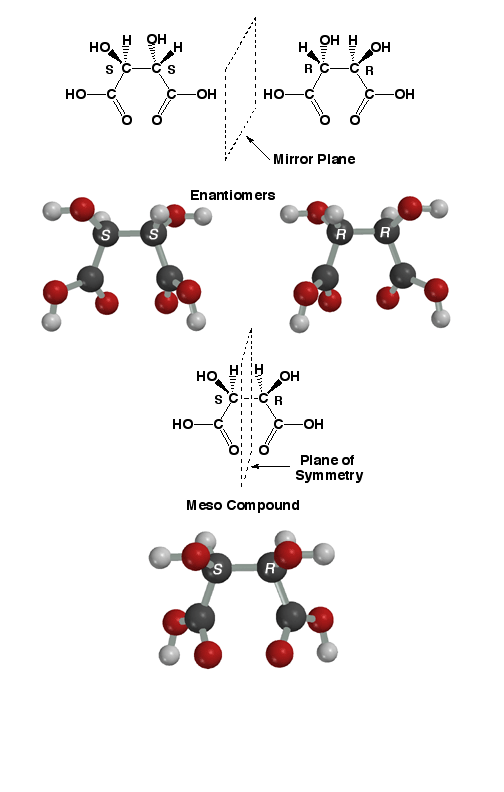
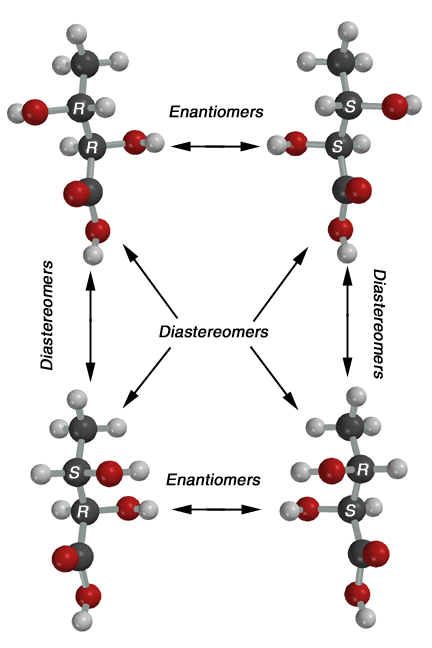
A
meso compound at first sounds like a contradiction. A meso compound is not
chiral even though it contains chiral centers. The key idea is that meso
compounds contain a plane of symmetry, so cannot be chiral. This occurs in
the special case that a molecule has chiral centers (in the above case 2
chiral centers) that have the same four substituents attached. In
these cases, the R,S/S,R stereoisomer will be a meso compound. As an example,
the tartaric acid isomers are shown about. The upper two are the S,S and
R,R stereoisomers, these are non-superimposable mirror images of each other
so they are enantiomers. There is no plane of symmetry in either of these
stereoisomers. On the other hand, the R,S/S,R stereoisomer shown below has
a plane of symmetry as indicated in the drawing. Thus, it is superimposable
on its mirror image (its mirror image is the same molecule), it is not chiral,
and the R,S stereoisomer is the same molecule as the S,R stereoisomer! The
bottom line is that symmetric molecules with two chiral centers like tartaric
acid only have three different stereoisomers, a pair of enantiomers (will
always be the R,R and S,S compounds) and a meso compound (will always be
the R,S/S,R stereoisomer).